Cancer Metastasis
Most cancer deaths are due to spreading of the primary tumor to one or several secondary sites, in a process called metastasis. These metastases are often more difficult to treat than the primary tumor and their presence marks severe progression of the disease. Tumor cell metastasis involves cell migration through heterogenous microenvironments in tissues, along anatomical features such as blood vessels and nerves, and into and out of vasculature. Mounting evidence has accumulated, demonstrating that metastatic tumor cells can migrate via several modes or mechanisms of migration, and can switch between these modes depending on the specific features of the microenvironment. Our research in this area is focused on understanding how distinct physical and biochemical cues from the tumor cell's microenvironment affect the phenotype, biological signaling, mechanical properties, and interactions with other cells during metastasis. We are interested in metastasis to the brain, which is especially difficult to treat and unique given that the process requires crossing of the blood-brain barrier.
Astrocytes are one type of neural cell that are in close proximity to (and/or in contact with) the brain microvasculature. We have recently shown that secreted factors by astrocytes from the brain microenvironment alter the migration, morphology, and cytoskeletal organization of metastatic breast tumor cells (see below). Check out our recent paper for more information.
We are also interested in how physical confinement affects tumor cell behavior and recently showed that bi-axial confinement in microchannels reduces cell divisions, leads to abnormal division events when cells do divide, and halts cell cycle progression at the S/G2/M phase.
Astrocytes are one type of neural cell that are in close proximity to (and/or in contact with) the brain microvasculature. We have recently shown that secreted factors by astrocytes from the brain microenvironment alter the migration, morphology, and cytoskeletal organization of metastatic breast tumor cells (see below). Check out our recent paper for more information.
We are also interested in how physical confinement affects tumor cell behavior and recently showed that bi-axial confinement in microchannels reduces cell divisions, leads to abnormal division events when cells do divide, and halts cell cycle progression at the S/G2/M phase.
|
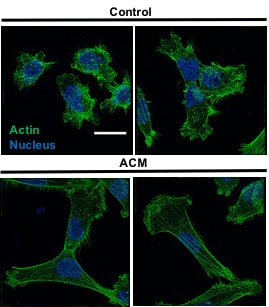
Figure to left from M.A. Shumakovich, C.P. Mencio, J.S. Siglin, R.A. Moriarty, H.M. Geller, and K.M. Stroka#. Astrocytes from the brain microenvironment alter migration and morphology of metastatic breast cancer cells, FASEB Journal 31:115049-5067 (2017). Confocal images reveal morphological and cytoskeletal alterations of MDA-MB-231 metastatic breast tumor cells in response to astrocyte conditioned media (ACM). These morphological changes are linked with increased migratory capacity. |
Also check out Dr. Stroka's previous work on cancer metastasis:
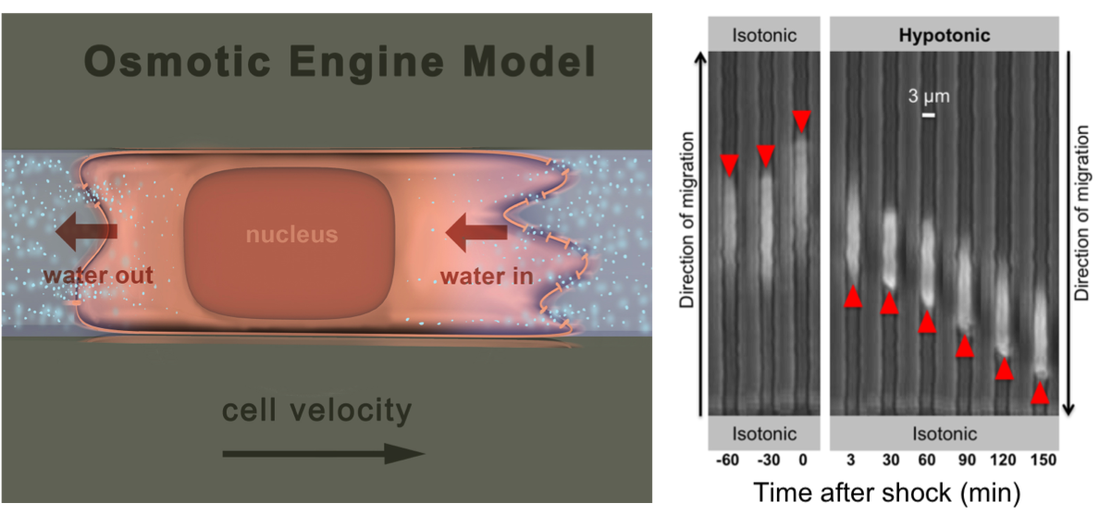
A new paradigm for Cell migration: THe Osmotic Engine Model
|
K.M. Stroka*, H. Jiang*, S-H Chen, Z. Tong, D. Wirtz, S.X. Sun, and K. Konstantopoulos, Water permeation drives tumor cell migration in confined microenvironments, Cell 157:1-13 (2014). (*, equal contribution). Selected for F1000Prime Pubmed ID: 24726433
K.M. Stroka, Z.Gu, S.X. Sun, and K. Konstantopoulos, Bioengineering paradigms for cell migration in confined microenvironments Current Opinion in Cell Biology 30C: 41-50 (2014) (Invited review). Pubmed ID: 24973724 |
PHYSICAL CUES GUIDE TUMOR CELL MIGRATION MECHANISMS
K.M. Stroka and K. Konstantopoulos, Physical cues guide tumor cell adhesion and migration, American Journal of Physiology - Cell Physiology 306: C98-C109 (2014) (Invited review). Pubmed ID: 24133064
P.S. Raman*, C.D. Paul*, K.M. Stroka, and K. Konstantopoulos, Probing cell traction forces in confined microenvironments, Lab on a Chip 13: 4599-4607 (2013). (*, equal contribution) Pubmed ID: 24100608
W.C. Hung, S-H Chen, C.D. Paul, K.M. Stroka, J.T. Yang, and K. Konstantopoulos. Distinct signaling mechanisms for cell migration in unconfined versus confined spaces, Journal of Cell Biology 202(5): 807-824 (2013). Selected for F1000Prime Pubmed ID: 23979717
E.M. Balzer, Z. Tong, C.D. Paul, W.-C. Hung, K.M. Stroka, A.E. Boggs, S.S. Martin, and K. Konstantopoulos. Physical confinement alters cell adhesion and migration phenotypes, FASEB Journal 26: 1-12 (2012). Pubmed ID: 22707566
P.S. Raman*, C.D. Paul*, K.M. Stroka, and K. Konstantopoulos, Probing cell traction forces in confined microenvironments, Lab on a Chip 13: 4599-4607 (2013). (*, equal contribution) Pubmed ID: 24100608
W.C. Hung, S-H Chen, C.D. Paul, K.M. Stroka, J.T. Yang, and K. Konstantopoulos. Distinct signaling mechanisms for cell migration in unconfined versus confined spaces, Journal of Cell Biology 202(5): 807-824 (2013). Selected for F1000Prime Pubmed ID: 23979717
E.M. Balzer, Z. Tong, C.D. Paul, W.-C. Hung, K.M. Stroka, A.E. Boggs, S.S. Martin, and K. Konstantopoulos. Physical confinement alters cell adhesion and migration phenotypes, FASEB Journal 26: 1-12 (2012). Pubmed ID: 22707566
Tumor cells interact with the vascular endothelium
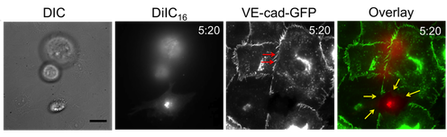
S. Hamilla*, K.M. Stroka*, and H. Aranda-Espinoza. VE-cadherin-independent cancer cell incorporation into the vascular endothelium precedes transmigration, PLOS ONE9(10): e109748 (2014). (*, equal contribution) Pubmed ID: 25275457
Giant obscurins are implicated in breast epithelial cell migration, metastasis, and mechanosensing
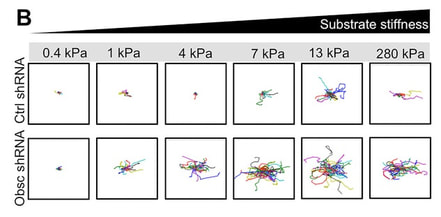
K.M. Stroka#, B.S. Wong, M. Shriver, J.M. Phillip, D. Wirtz, A. Kontrogianni, and K. Konstantopoulos#. Loss of giant obscurins alters breast epithelial cell mechanosensing of matrix stiffness, Oncotarget DOI: 10.18632/oncotarget.10997 (2016). Pubmed ID: 27494856
M. Shriver, K.M. Stroka, M. Vitolo, S.S. Martin, D. Huso, K. Konstantopoulos, and A. Kontrogianni. Loss of giant obscurins from breast epithelium promotes cell migration, invasion, and metastasis, Oncogene 34:4248-4259 (2015). Pubmed ID: 25381817
M. Shriver, K.M. Stroka, M. Vitolo, S.S. Martin, D. Huso, K. Konstantopoulos, and A. Kontrogianni. Loss of giant obscurins from breast epithelium promotes cell migration, invasion, and metastasis, Oncogene 34:4248-4259 (2015). Pubmed ID: 25381817