Vascular Mechanobiology
The vascular endothelium lines all blood vessels in the human body and creates a barrier for cells, molecules, and ions to actively or passively move from the bloodstream into tissues, or from tissues into the bloodstream. Transport across the vascular endothelium may be a naturally occurring process, such as in the instance of the innate immune response, where neutrophils transmigrate through endothelial cells, out of the blood vessel, and into nearby tissue to attack foreign material (e.g., check out Dr. Stroka's previous work on neutrophil transmigration). Or, transport across the endothelium may occur in undesired situations, such as when circulating tumor cells, originally from a primary tumor, exit the blood stream during metastasis. Furthermore, transport across the vascular endothelium may be an important hurdle to overcome when designing drugs that are injected intravenously and must cross vascular barriers to be effective in a target tissue. In all cases, the vascular endothelial cells must respond to an array of mechanical forces from the microenvironment, and they also undergo changes in biomechanical properties, which may lead to alterations in homeostasis of the blood vessel.
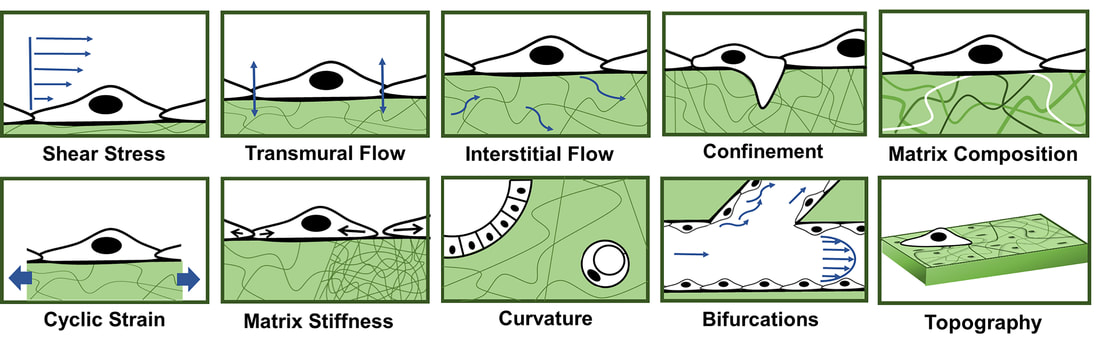
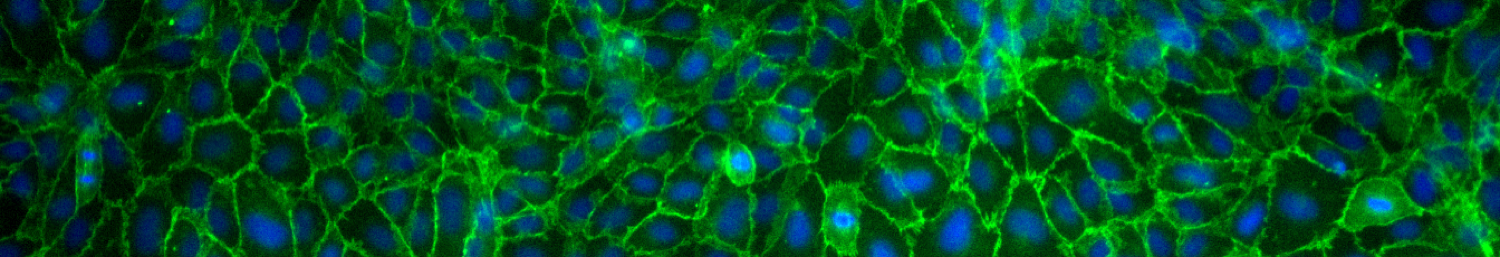
A recent review paper published by our lab highlights the array of mechanical forces sensed by the vascular endothelium and how application of these forces leads to alterations in biological signaling and transport across the vasculature. Our research efforts in this area are currently aimed at understanding the biological response of brain microvascular endothelial cells to various biomechanical cues (see schematic below), and how this information can be used towards development of an in vitro blood-brain barrier model. In addition, we are investigating the mechanobiology of interactions between metastatic tumor cells and the vascular endothelium. We are concurrently developing software to quantitatively assess the morphology of cell-cell junctions in the vascular endothelium. Our recent publication highlights our research in this area and we have made the Junction Analyzer Program freely available for download on our Github site.
A recent review paper published by our lab highlights the array of mechanical forces sensed by the vascular endothelium and how application of these forces leads to alterations in biological signaling and transport across the vasculature. Our research efforts in this area are currently aimed at understanding the biological response of brain microvascular endothelial cells to various biomechanical cues (see schematic below), and how this information can be used towards development of an in vitro blood-brain barrier model. In addition, we are investigating the mechanobiology of interactions between metastatic tumor cells and the vascular endothelium. We are concurrently developing software to quantitatively assess the morphology of cell-cell junctions in the vascular endothelium. Our recent publication highlights our research in this area and we have made the Junction Analyzer Program freely available for download on our Github site.
Figure above from Gray and Stroka, Seminars in Cell and Developmental Biology 71:106-117 (2017).
Relevant publications:
Relevant publications:
- M.A. Pranda*, K.M. Gray*, G.M. Dawson, A.J.L. DeCastro, J.W. Jung, and K.M. Stroka#. Tumor cell mechanosensing during incorporation into the brain microvascular endothelium, Cellular and Molecular Bioengineering (in press, 2019). (*, equal contribution). Link *** invited as part of Cellular and Molecular Bioengineering Young Innovator Award special issue.
- K.M. Gray, D.B. Katz, E.G. Brown, and K.M. Stroka#. Quantitative phenotyping of cell-cell junctions to evaluate ZO-1 presentation in brain endothelial cells, Annals of Biomedical Engineering 47(7): 1675-1687 (2019). Link
- K.M. Gray and K.M. Stroka#. Vascular endothelial cell mechanosensing: New insights gained from biomimetic microfluidic models, Seminars in Cell and Developmental Biology 71:106-117 (2017). Link *** invited review article for special issue on “Mechanosensing: from Molecules to Tissues”
- K.M. Stroka, J.A. Vaitkus, and H. Aranda-Espinoza. Endothelial cells undergo morphological, dynamical, and biomechanical changes in response to TNF-α, European Biophysics Journal 41(11): 939-947 (2012). Pubmed ID: 22940754
- K.M. Stroka and H. Aranda-Espinoza. Effects of morphology versus cell-cell interactions on endothelial cell stiffness, Cellular and Molecular Bioengineering 4(1): 9-27 (2011). Pubmed ID: 21359128
<-- Back to Research page.